Source: European Pharmaceutical Manufacturer - Dr Rolf Abelman, Najet Mebaki - June 2021.
Najet Mebarki - senior product marketing manager at SGD Pharma and Dr Rolf Abelman - managing direction at IVM Childsafe explore how the pharma industry has continued to advance its efforts to improve the safety of child-resistant packaging.

Unintentional poisoning continues to be a widespread medical emergency across the globe, with medication being one of the most common causes, putting children at the highest risk of potentially fatal accidents. In the European Union (EU), poisoning is the fifth leading cause of unintentional death among children and adolescents. Research from the European Child Safety Alliance estimates that 3,000 children under the age of 14 die from acute poisoning every year. More recently, this has sparked increased efforts into improving parental education in ‘child-proofing’ homes, as well as patients and stakeholders calling on pharmaceutical companies to recognise the importance of child-resistant closures (CRCs) in medical packaging. The reduction in the rates of child poisoning since 2010 in the United States (US) is indicative of a global decrease (Figure 1), but accidents can and do still occur, and regulations are continually evolving to optimise the safety of child-resistant packaging for drugs.
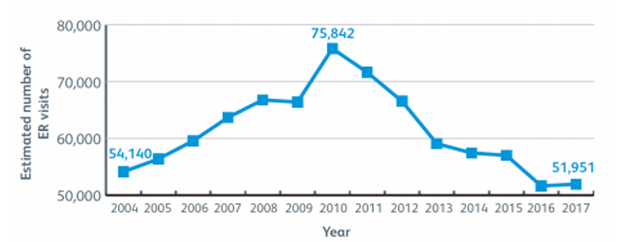
Figure 1: Estimated number of emergency room (ER) visits in the United States for accidental medicine poisoning under the age of 6 years. (Source: Safekids Worldwide).
As the industry is improving its packaging standards and unintentional child poisoning therefore decreases, it continues to be a prominent factor in the development of pharmaceutical packaging. Effective CRCs for medical packaging must protect young children from gaining access to harmful substances, without hindering the ability of older patients to access their medication.
Global guidance and regulations
There are varied mechanisms for incorporating CRCs on medical packaging, which are broadly categorised into:
re-closable bottles, which include push and turn caps or squeeze and turn caps.
non-re-closable blister packs which include a peel and push method.
Now, in many countries, pharmaceutical and healthcare companies are required by law to produce packaging that is child resistant. The US has been leading the way in child-resistant packaging for years, where protocols were originally established in 1966. In 1970, the Poison Prevention Packaging Act (PPPA) was passed and placed under the jurisdiction of the Food and Drug Administration (FDA). In addition, the International Organisation for Standardisation (ISO) has published an internationally recognised agreed standard test procedure for re-closable child-resistant packaging and in Europe, several regulations have been introduced to complement this ISO standard.
For packaging to be classed as child-resistant, it must meet one of the following standards:
ISO 8317:2015 – the International standard
EN 14375:2018 – the European standard
US 16 CFR § 1700.20 – US regulation
To qualify as CRC packaging, pharmaceutical companies must now have certification of child resistance for their full packaging solution – including the container and closure – and must recertify if either component is changed. One barrier that drug manufacturers face is the time and cost associated with certification. By partnering with a packaging manufacturer that can provide innovative packaging, as well as the required certification, this process can be streamlined, and companies can ensure child safety for their drugs.
As awareness of unintentional child poisoning increases globally, large pharmaceutical companies are driving recognition and innovation in child-resistant packaging, with more advanced designs every year. One example is GlaxoSmithKline (GSK) which, as one of the world’s largest pharma companies, has made significant contributions to increasing awareness of the importance of child-resistant packaging and has integrated this approach into its drug manufacturing pipeline.
However, there are important considerations when advancing packaging designs, such as the cost and sustainability. For example, failure to comply with exact requirements of child-resistant packaging could result in company fines or payouts in the event of child injury, while brand damage is also at risk in the event of unintentional poisoning. In terms of sustainability, increasing environmental awareness across the global pharmaceutical industry has drawn attention to the need for more sustainable packaging solutions. Aligning child-resistant packaging with additional components that make these solutions sustainable are increasingly important for both customers and company initiatives alike. Moreover, innovative user-friendly child-resistant packaging solutions are driving the move to a more patient-centered treatment approach.
Senior-friendly packaging
When developing child-resistant packaging for pharmaceuticals, one of the greatest challenges is designing a closure that both prevents children from gaining access to harmful substances, without limiting the ability of senior patients to take their medication. Increasing population age combined with the growing trend for home care is directing the focus of child-resistant packaging simultaneously onto the accessibility needs of older patients. There has been criticism over the lack of acknowledgement in current protocols for disabled and vulnerable patients, who, if their needs are not met, may leave the closure off their medication and, in turn, increase the risk of child access.
This, therefore, provides an opportunity for pharmaceutical packaging manufacturers and pharmaceutical companies to lead the industry in designing truly child-resistant senior-friendly (CRSF) packaging for medicines. Strong relationships between drug manufacturers and packaging suppliers, as well as with healthcare providers and patients, will help facilitate this approach, for example by overcoming the currently perceived trade-off between child resistance and senior-friendly packaging.
The future with safe, compliant child-resistant packaging
Following the introduction of child-resistant packaging requirements, the incidence of poisoning in children has decreased steadily throughout the last 10 years. As well as enhanced regulation, this decrease is thought to be attributed to increasing awareness campaigns and educational efforts, through initiatives such as the Centers for Disease Control and Prevention (CDC) PROTECT Initiative and Up and Away Campaign, and Safe Kids Worldwide’s medicine safety programme.
However, unintentional poisonings and fatalities still occur across the globe. The risk to children depends heavily on their environment, and with more adults receiving prescriptions for medication such as sleeping aids, strong painkillers, and antidepressants, childhood exposure to harmful medication increases.
As certain drugs have become more problematic for child safety, such as the increased prescription of opioids, the need for safe, compliant child-resistant packaging has received increased attention. There is always room to improve the safety of pharmaceutical packaging that also maintains medication compliance with patients, particularly seniors. Innovation is continuing to take place to ensure child-resistant packaging is truly senior-friendly. As we look towards the future, it is vital that drug manufacturers and pharmaceutical packaging manufacturers collaborate to supply the safest packaging solutions and meet the regulatory requirements to prevent unintentional deaths across the world.



































